What is Stalpex?
Stalpex contains 50/500 mcg salmeterol xinafoate/
fluticasone propionate dose inhalation powder.
Indications 2
Asthma
-
Stalpex is indicated for use in patients with severe asthma 12 years of age and older only
-
Regular treatment of patients with severe asthma, where use of a combination product (long-acting ß2 agonist and inhaled corticosteroid) is appropriate:
- - patients not adequately controlled on a lower strength corticosteroid combination product, or
- - patients already adequately controlled on an inhaled corticosteroid in a high strength and a long-acting ß2 agonist
COPD
-
Symptomatic treatment of patients with a FEV1 <60% predicted normal (pre-bronchodilator) and a history of repeated exacerbations, with significant symptoms despite regular bronchodilator therapy
Dosing 2
Asthma
-
One inhalation twice daily. Once control of asthma is attained, treatment should be reviewed and consideration given to whether patients should be stepped down to a lower dose ICS/LABA combination or ICS alone
COPD
-
One inhalation twice daily
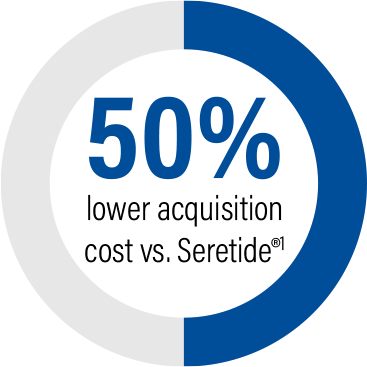
Stalpex acquisition cost
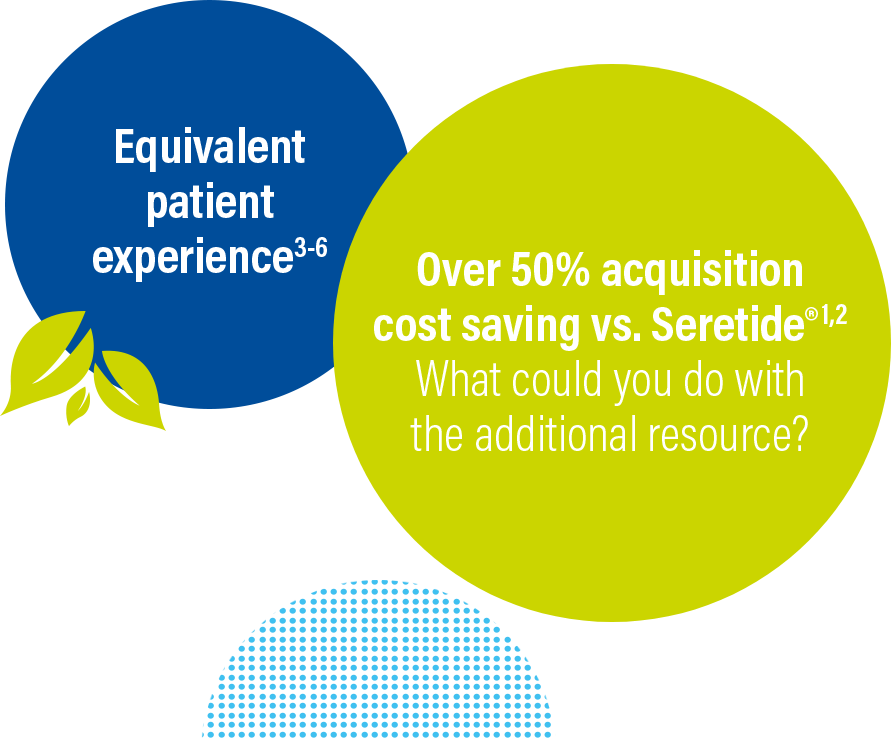
Stalpex offers acquisition cost savings of
over 50%
vs. the equivalent Seretide® pack.
1
One of the lowest costs of all dry powder inhaler salmeterol/fluticasone propionate 50/500 products.1
Sources: BNF March 20231
Prices correct at time of development but are subject to change without prior notice. E&OE.
Think Stalpex for all suitable salmeterol xinafoate/fluticasone propionate prescriptions
Prescribe Stalpex by brand to ensure:
-

Your patients get the device
prescribed -

You get the full acquisition cost savings for
your practice -

Your health economy gets the full
acquisition cost savings
Why choose Stalpex?
Stalpex 50/500 mcg offers an equivalent user
experience to Seretide® Accuhaler®2-5
-
The same active substance as Seretide® Accuhaler®2,4,5
-
Equivalent strength to 50/500 mcg Seretide® Accuhaler® dose4
-
A functionally equivalent DPI device3
-
Stalpex has been shown to deliver an equivalent dose to Seretide® Accuhaler® in equivalence studies4
…branching out in a
cost-conscious direction

Support for payers
Glenmark can provide you with estimates of financial savings for your health economy. To find out how much your health authority could save, click on the link below to arrange an appointment.
Contact UsInspired thinking by Glenmark Respiratory
We’re proud of our work designed to make a difference in respiratory medicine. Visit our site to find out more and access free education resources.
Explore nowThe Glenmark planned approach –
commitment to supply readiness
We are committed to ensuring Stalpex is available to order
from the moment a new customer comes on board:
Support for patients
When you prescribe Stalpex by brand, your patients have access to clear information and support to help ensure they take their medication correctly.
The Stalpex dedicated patient website contains information and support for patients prescribed Stalpex. Here they will find guidance on how to use their inhaler, answers to FAQs and links to trusted websites.
Click here to view.

View the Stalpex demonstration video
You can use it to facilitate Stalpex device training with your patients.
The video can be accessed directly by patients prescribed Stalpex on the patient site.